Human body plug and play

2018.12.08
One of the most perplexing issues to confront modern medicine has been the successful transplantation of human organs.
The problem isn’t so much the procedure – although there is always the risk of the recipient’s body rejecting the organ, hence the use of immunosuppressive drugs – but the fact that demand vastly outstrips supply. Transplant patients get placed on a waiting list for an organ, making the success of the system entirely dependent on the number of organ donors. The donor has to have the right blood type and the organ must be the right size, for there to be a match. Many patients become sicker as they wait; in every country, hundreds die each year waiting for an organ.
While the first organ transplant was believed to have occurred as far back as the third century AD – when the leg of an Ethiopian Moor was grafted onto a white Roman as depicted in a Renaissance painting – it wasn’t until the dawn of the 20th century that science had progressed to a point where doctors were confident in performing the procedure.
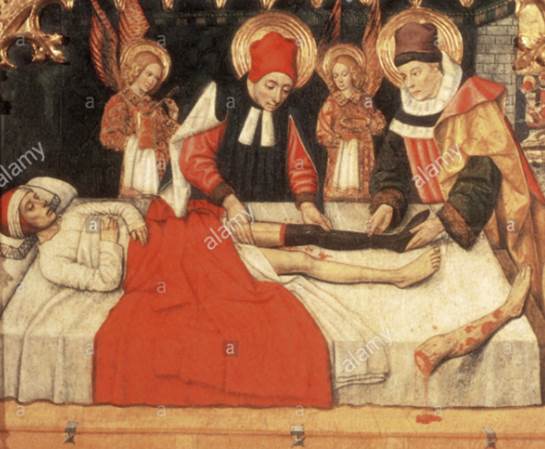
Among the first successes were the emigration of a cornea from an 11-year-old boy into a farm laborer whose eyes had been damaged while slaking lime; and skin grafts on British soldiers during World War I by Sir Harold Gillies, said to be the father of plastic surgery.
Organs that can now be transplanted include the liver, kidney, pancreas, heart, lung, intestine, cornea, middle ear, skin, bone, bone marrow, heart valves, and connective tissue.
The first successful major organ transplant was in 1954, when a Massachusetts man donated a healthy organ to his twin brother who had chronic nefritis, a condition that inflames the kidney. That was followed by the first pancreas transplant in 1966 and the first heart and liver transplants a year later. In 1984 a national registry for organ matching was created, however the system is imperfect to say the least, with long waiting lists for donors.
According to the United Network for Organ Sharing, every day 21 people in the United States die waiting for an organ, and over 120,000 people are on organ transplant waiting lists.
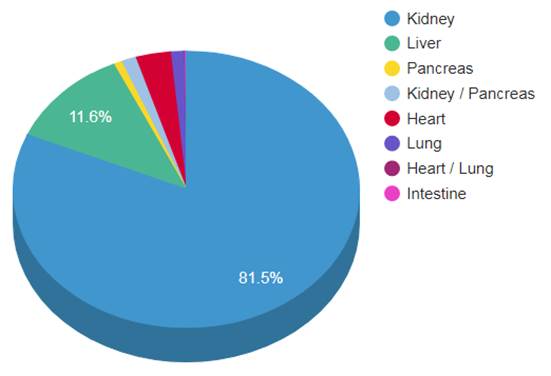
The situation is worse in Canada. While Spain has 43 donors per million people, the US has 26, Britain has 21, and Canada has just 20. Out of 4,500 Canadians waiting for an organ, about 260 will die each year, according to The Organ Project. That’s five deaths per week. A few more factoids about organ transplants in Canada:
- Only 1% of deaths in hospitals yield organs for transplants
- 75% of the waiting list are kidney recipients. The next in-demand organs are the liver, lungs and heart
- Ontario is estimated to spend $100 million a year caring for sick patients awaiting a kidney transplant
- The average kidney transplant patient will wait four years
- Over 1,600 people are added to waiting lists each year
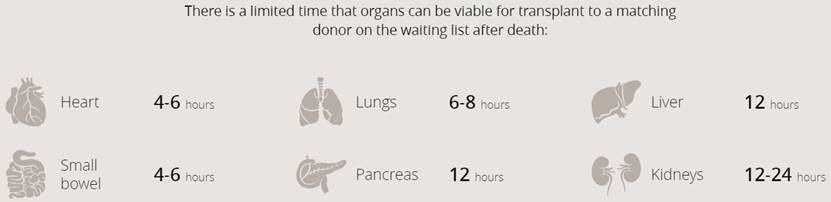
Imagine if, instead of waiting for an organ from another person – possibly a relative but likely a stranger – you could walk into a doctor’s office and have one manufactured, with your cells. It sounds far-fetched, but the technology now exists for the tailor-made transplantation of organs through brand-new medicine called 3D bioprinting.
What is 3D bioprinting?
Most people are familiar with the relatively new field of 3D printing. Put simply, 3D printing is a progression of 2D printing, where a third dimension is added to the printing of images on a flat surface (a regular ink-jet printer), adding depth and allowing the printer cartridge to move in all directions. A digital file is first created using modeling software, then sent to the printer, depositing layers of the chosen material – often plastic or wax – to build up the final product.
Formerly known as stereolithography, 3D printing was invented in 1983 by Chuck Hull, co-founder of 3D Systems. It took over 30 years for the technology to become mainstream, but now 3D printing can be done by anyone with access to a 3D printer, which can be purchased for under $500.
Among the more interesting items that have been 3D-printed are prosthetic limbs, fabricated firearms, electrical vehicles, steel parts (Caterpillar introduced the first 3D-printed excavator in 2017), quick-build homes, parts for combat aircraft and spacecraft, and even decorative chocolates.

NASA 3d prints food – autodesk.com

Bioprinting operates on the same general principle as regular 3D printing but instead of plastic, wax or other matter, bioprinters deposits layers of living cells to build structures like blood vessels or skin tissue. The cells are taken from an animal or a human being and cultivated until there are enough to create “bio-ink” which is then loaded into the printer using mechanical syringes. Adult stem cells can also be utilized.
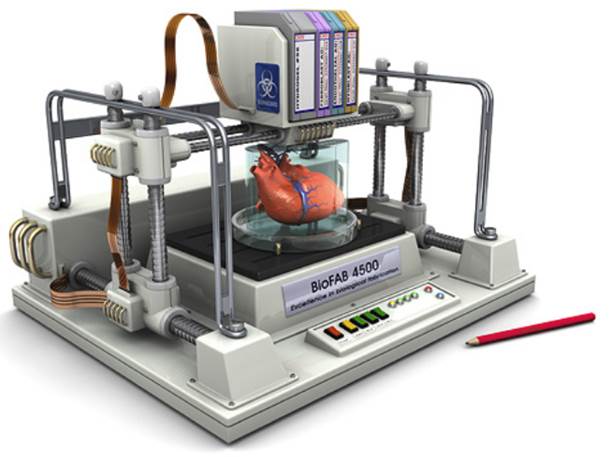
Key to the process is a dissolvable gel which acts as a kind of incubator for the cells to multiply – like an embryo growing in a womb. Researchers may also plant cells around 3D scaffolds made of biodegradable polymers or collagen, allowing them to develop into functional tissue. The cells use their inherent properties to seek out similar cells to join with. Researchers are able to control the shape into which the cells form, and the printer builds the final structure.
After the tissues are fully grown and shaped, they are placed into a recipient’s body. The hope is that the 3D-printed object becomes as much a part of the patient’s body as the cells he or she was born with.
Dr. Utkan Demirci, professor at Stanford University School of Medicine, defines bioprinting as the process of using advanced additive manufacturing technologies to pattern biological materials, such as cells, biomaterials and biomolecules, for the fabrication of tissue-mimicking constructs. This novel approach requires biocompatible materials called bio-inks to act as the matrices for printed cells, which can then be grown in bioreactors to further develop and become functionally mature. PhysicsWorld: The past, present and future of 3D bioprinting
There are currently five common methods of 3D bioprinting:
- Inkjet bioprinting: Droplets of bio-ink are deposited, layer by layer, onto a culture plate. Cells that can help fight breast cancer have been successful printed using inkjet bioprinting.
- Extrusion bioprinting: Polymer or hydrogel is loaded in syringes and dispensed via pneumatic- or screw-driven force, onto a building platform. The motion is controlled by a computer. Extrusion bioprinting offers lower resolution than inkjet bioprinting but the fabrication speed is considerably higher, allowing anatomically-shaped objects to be generated.
- Laser-assisted bioprinting: A laser is used to deposit the biomaterials into a receptor via a tape covered with biological material. The laser irradiates the tape, causing the biological material to evaporate and reach the receptor in the form of droplets. The droplets contain a biopolymer that acts as an adhesive to help the cells to grow. This high-resolution bioprinting method is being used in a partnership between French bioprinting company Poietis and L’Oréal to recreate a hair follicle that could lead to a cure for baldness.
- Stereolithography: Stereolithographic bioprinting uses a “digital micromirror” to direct ultraviolet light onto the printing surface. Light directed by the micromirrors triggers the formation of molecular bonds, which cause light-sensitive hydrogels to form into solid material.
- Bioprinting with acoustic waves: Using a device that allows cells to be manipulated with acoustic waves, researchers can manipulate where the waves will meet along three axes. The waves then form a trap that captures the cells, which are collected to create 3D patterns.
How far has it progressed?
Some of the most advanced work on bioprinting has been done at the Wake Forest Institute for Regenerative Medicine in California. One of the first major structures that Wake Forest bioprinted was a human bladder. Made from cells extracted from a patient with a poor-functioning bladder, the 3D-printed bladder was successfully transplanted. The project built on custom-grown bladders that had previously been transplanted into seven patients suffering from spina bifida, a birth defect that affects the spinal cord.
Wake Forest staffers have also created an outer human ear, and implanted bioprinted skin, bone and muscle on laboratory animals that successfully grew into surrounding tissue.
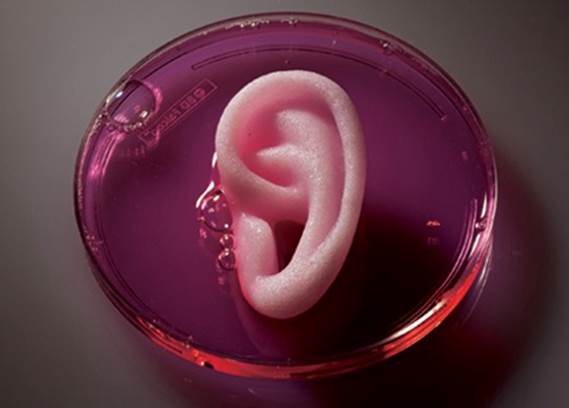
But these are just the early prototypes. Bioprinting’s leading researchers expect that as the field develops, the technology will evolve into the ability to bioprint major organs for transplant into human donors – completely eliminating waiting lists, patient deaths, and dramatically increasing the success rate of organ transplants.
The institute’s director, Anthony Atala, sees bioprinting as totally transforming the relationship between the transplant patient and doctor, in much the same way that Dell changed the way consumers interacted with the computer company that sold PCs tailored to each customer’s unique needs. Patients could order replacement parts in much the same way they might order a new clutch for their Mazda.
“You’d have companies that exist to process cells, create constructs, tissue. Your surgeon might take a CT scan and a tissue sample and ship it to that company,” Atala said in a feature article on bioprinting in Smithsonian Magazine.
The company would then ship the organ back a week or so later, ready for implantation. Welcome to the new world of regenerative medicine: the plug and play human body.
Atala said the technology is developing to the point where researchers are almost able to replicate simple organs like the outer ear and the trachea (windpipe). Importantly, there are no real surgical challenges, he told Smithsonian.
Wake Forest is also working on a skin-cell printer capable of printing living skin cells directly onto a patient. Funded by the US Defense Department, the project is targeted at veterans who have sustained facial or genital injuries in battle. The wound is scanned to get the exact size and shape, then the cells are bioprinted to fit the wound. In 2015 clinical trials were expected to take place in five years pending FDA approval. In 2017 scientists in Madrid created a prototype of a 3D bioprinter that can create functional human skin. The printer is adequate for transplanting skin and for testing cosmetic, chemical and pharmaceutical products, ScienceDaily reported.
Other centers of research, like the Texas Heart Institute in Houston, aren’t trying to bioprint structures from scratch; rather, they use existing ones. An example is decelluarized pig hearts. The organs have been stripped of muscle and other living tissue, but the original architecture is intact. The idea is to use decelluarized pig hearts, repopulated with bioprinted human cells, for implantation into humans. So far the institute has succeeded in injecting pig hearts with living bovine cells, then inserted them into cows where they worked successfully next to a cow’s heart.
Already, patients with a defective heart valve can have a pig’s valve or a mechanical valve implanted. Doris Taylor, director of the institute’s regenerative medicine research program, says the decelluarized method gets around the tricky process of printing at the extremely high resolution required for highly vascularized (containing many blood vessels) organs like the heart.
“The tech is going to have to improve a great deal before we’re able to bioprint a kidney or a heart, and get blood to it, and keep it alive,” Taylor told Smithsonian.
More recent developments though are moving in that direction. In 2016 Harvard researchers 3D-printed the first “heart-on-a-chip”. The tiny device contains living human heart cells that mimic the heart’s functions.
This past June, 3D printing startup BioLife4D successfully produced human tissue in the form of a cardiac patch – derived from a patient’s white blood cells with multiple cell types contained in the human heart. According to pharmaforum, it’s another step towards bioprinting major organs for transplant.
Northwestern University in Illinois debuted a 3D printed ovary using the acoustic waves method described above, and in Sweden, researchers have successfully created human cartilage tissue, also using acoustic waves.
Very recently, Russian scientists aboard the International Space Station successful bioprinted the first organ in space: a mouse’s thyroid. Space’s zero-gravity environment enables organs and tissues to mature faster than on Earth.
What are the challenges?
The holy grail of 3D bioprinting would be to come up with a viable kidney for transplant. Prof. Atala, of the Wake Forest Institute, created the first small-scale bioprinted kidney in 2002. However, Atala is the first to admit that his machine-produced kidney is nowhere near at the level it needs to be for a human transplant. A TED Talk Atala gave in 2011 about bioprinting, which culminated with a dramatic display of an object – really an over-sized bean – became controversial when the press got ahold of it and printed enthusiastic, but wrong, stories about the technology eliminating the need for a kidney transplant.

Describing the complexity of a human kidney, The Smithsonian Magazine article indicates why bioprinting still has a ways to go:
Sit down with a pencil and a piece of paper and you could hardly dream up something more architecturally or functionally complex than the human kidney. The interior of the fist-size organ is made up of solid tissues traversed by an intricate highway system of blood vessels, which measure as little as 0.010 millimeters in diameter, and approximately a million tiny filters known as nephrons, which send healthful fluids back into the bloodstream and waste down to the bladder in the form of urine. To bioprint a kidney, you’d have to be able to cultivate and introduce not only functioning kidney cells and nephrons, you’d also need to have mastered how to populate the organ with a vasculature to keep the organ fed with the blood and nutrients it needs. And you’d have to build it all from the inside out.
Another potential roadblock is the cost. No-one yet knows what it would cost to bioprint and transplant a human organ on demand, and how accessible the procedure would be to the masses of patients requiring a transplant. And while there have been successful bioprinted organ transplants, there haven’t been enough to determine how well the human body will accept the new tissue or artificial organ.
Finally, one shouldn’t underestimate the complexity and level of difficulty involved. As pharmaforum points out, “A complex network of cells, tissues, nerves and structures in a human organ need to be correctly positioned with a highest precision for it to function properly. From arranging the thousands of tiny capillaries in a liver, to printing a heart that beats, it is a long, difficult process.
While some parts of the human body are more complex than others, each piece has its own specialised requirements and issues that need addressing. The selection of the right materials, cell types and bio-inks must be as precise as the blueprint itself.”
The website estimates that bioprinting of fully functioning, complex organs like hearts, kidneys and livers is at least 10 years away.
In the meantime, patients may need to be satisfied with machine-engineered tissue rather than organs, according to The Medical Futurist:
Synthetic skin, a bionic ear, bladder, or cornea might be the first tissues to be either bioprinted or grown in the lab on demand – since they are tissues containing a small number of cell types. After that, more complicated ones might be engineered.
Investment opportunity
Still, 3D bioprinting has come a long way since Atala’s first artificial bladder in 2002. At Ahead of the Herd, we think it is the next big thing in regenerative medicine. Science always starts out with experimentation, sometimes many years of it, before the technologies are commercialized. We want our subscribers to be well aware of 3D bioprinting’s potential, putting them in a position to get in early to companies that are offering bioprinted products.
While there are currently a handful of bioprinting firms, we see an entire ecosystem of small firms developing, with each focusing on a different aspect, technology or part of the body. It will not take 10 years for start up pubco’s to IPO seeking money to develop their technologies.
According to BIS Research, within the next seven years, the bioprinting market is expected to expand by 15.7%, and by 2025, it will be worth $4.7 billion. The United States and Canada are the industry leaders, making bioprinting an ideal new sector for North America-focused investors.
Conclusion
3D bioprinting could represent the solution to the transplant dilemma that has plagued medicine for hundreds of years. As I write this, people are dying waiting for organs, or in severe pain. Imagine if that pain could go away, literally, in a heartbeat. While it’s still early days as far as getting to the transplantation of major organs, the potential for this technology is enormous. Instead of patients being hardwired into an organ transplant system that is slow, risky and heartbreaking for those who are too far down the waiting list, 3D bioprinting holds the promise of medicine tailored to the individual. We are on the cusp of the human body being plug and play: get a scan of your defective organ, and a tissue sample, then send it off to a company for organ fabrication. A week later, presto! New organ, problem solved.
3D bioprinting is high on my radar as I hunt for new ahead of the herd investment opportunities. Is it on yours? If not perhaps it should be.
Richard (Rick) Mills
Ahead of the Herd is on Twitter
Ahead of the Herd is now on FaceBook
Ahead of the Herd is now on YouTube
Legal Notice / Disclaimer
This document is not and should not be construed as an offer to sell or the solicitation of an offer to purchase or subscribe for any investment.
Richard Mills has based this document on information obtained from sources he believes to be reliable but which has not been independently verified.
Richard Mills makes no guarantee, representation or warranty and accepts no responsibility or liability as to its accuracy or completeness. Expressions of opinion are those of Richard Mills only and are subject to change without notice. Richard Mills assumes no warranty, liability or guarantee for the current relevance, correctness or completeness of any information provided within this Report and will not be held liable for the consequence of reliance upon any opinion or statement contained herein or any omission.
Furthermore, I, Richard Mills, assume no liability for any direct or indirect loss or damage or, in particular, for lost profit, which you may incur as a result of the use and existence of the information provided within this Report.
Legal Notice / Disclaimer
Ahead of the Herd newsletter, aheadoftheherd.com, hereafter known as AOTH.Please read the entire Disclaimer carefully before you use this website or read the newsletter. If you do not agree to all the AOTH/Richard Mills Disclaimer, do not access/read this website/newsletter/article, or any of its pages. By reading/using this AOTH/Richard Mills website/newsletter/article, and whether you actually read this Disclaimer, you are deemed to have accepted it.